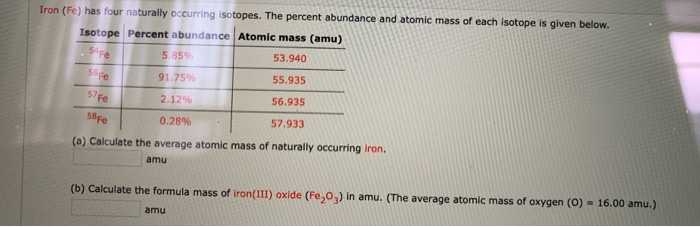
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.

The atomic mass of metal M is 56. Calculate the empirical formula of its oxide containing 70% of M. from Chemistry Some Basic Concepts of Chemistry Class 11 CBSE

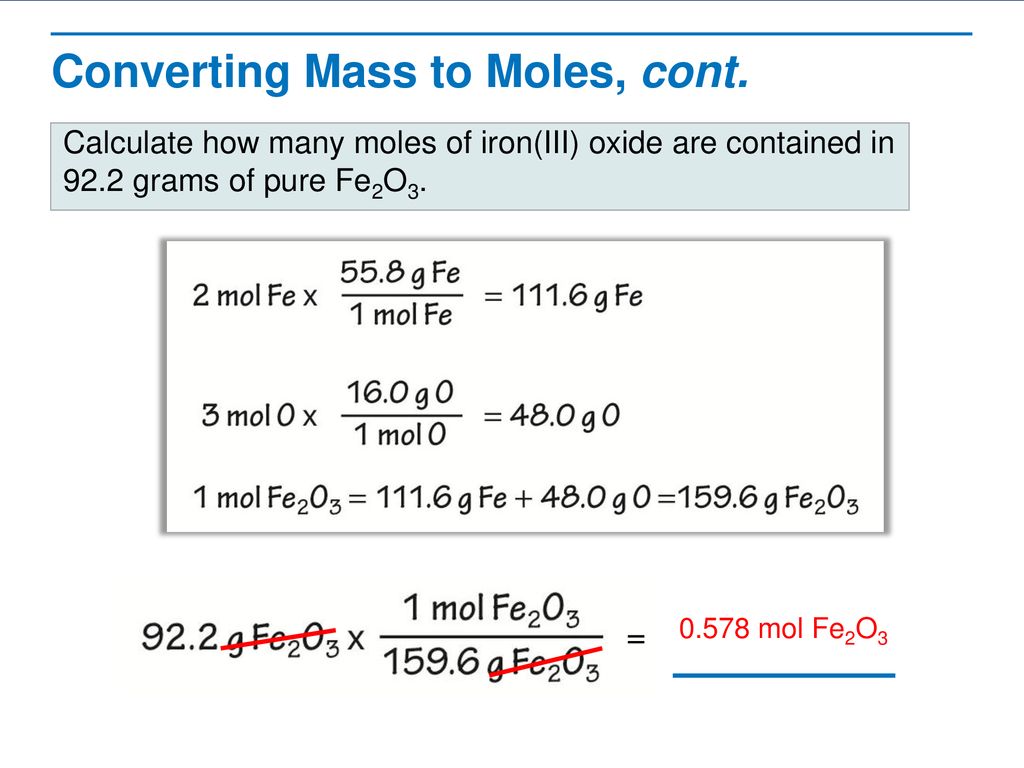
Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively.

Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that molecular mass is 159.69g.

determine the molecular formula of an oxide of iron , which has 69.9% iron and 30.1% dioxygen by mass(Given - Brainly.in

Using the balanced equation and their knowledge of the atomic masses of Fe and O, the scientist calculates - Brainly.com
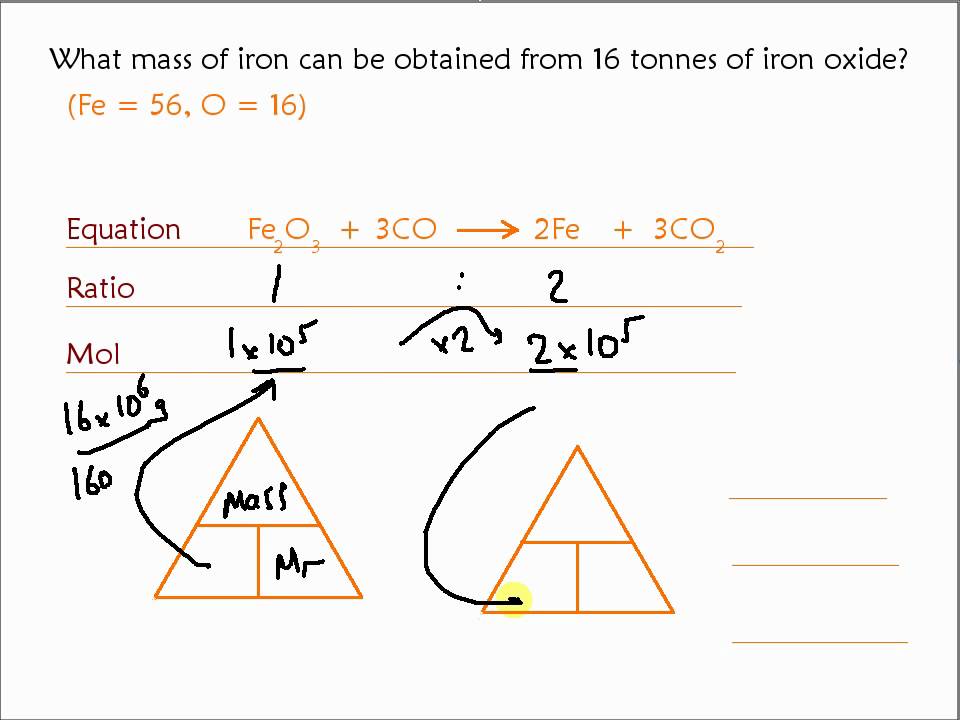
How to answer this question? The formula of the iron oxide is Fe2O3. What is the maximum mass of iron that can be obtained from 240 tonnes of iron oxide, Fe2O3 (relative

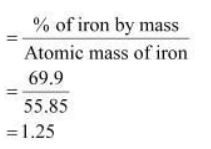





![Tamil] Determine the empirical formula of an oxide of iron which has Tamil] Determine the empirical formula of an oxide of iron which has](https://d10lpgp6xz60nq.cloudfront.net/physics_images/FM_CHE_XI_V01_C01_E04_164_S01.png)